Abstract
Background: Lisocabtagene maraleucel (liso-cel), an autologous CD19-directed 4-1BB-costimulated chimeric antigen receptor (CAR) T-cell therapy, has demonstrated high overall response rates in patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL). However, approximately half of the treated patients will fail to achieve remission or will relapse. Thus, strategies to further improve the efficacy of CD19 CAR-T cell products are needed. NKTR-255 is a novel clinical grade polymer-conjugated interleukin-15 (IL-15) agonist, which engages the entire IL-15 receptor complex with improved pharmacokinetic properties compared with native IL-15, further promoting the proliferation and survival of T cells. In patients treated with CD19 CAR-T cells, peak serum IL-15 concentration after CAR-T cell infusion was associated with CAR-T cell counts in blood and antitumor response. Furthermore, preclinical data in immunodeficient mice bearing Raji lymphoma have shown that NKTR-255 enhanced expansion, survival, and anti-tumor activity of CD19 CAR-T cells. These results suggest that IL-15 supplementation may enhance efficacy of CD19-directed CAR-T cell therapy.
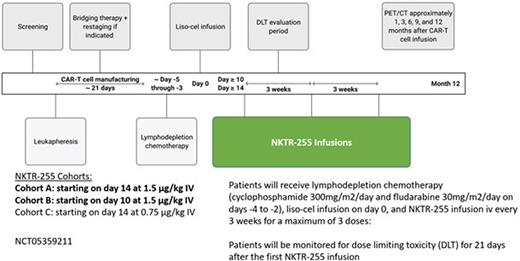
Methods: NKTR-255 is being evaluated in combination with the CD19 CAR-T cell product, liso-cel, in an open-label, single center non-randomized study (phase 1b) in patients with R/R LBCL who are eligible for commercial liso-cel treatment. Subjects will receive lymphodepletion chemotherapy with cyclophosphamide and fludarabine followed by liso-cel infusion per Breyanzi® package insert, and NKTR-255 at 1.5 µg/kg intravenously every 3 weeks starting at approximately 10 to 14 days after CAR-T cell infusion. We considered that the earliest suitable time after CAR-T cell infusion to start NKTR-255 in this initial clinical trial would be around day 7 to 14, to allow deferral of patients with severe CAR-T cell related toxicities, namely cytokine release syndrome and immune effector cell neurotoxicity syndrome, early after CAR-T cell infusion and decrease potential toxicities from IL-15 supplementation. Approximately 12 subjects will be enrolled during dose escalation to determine the optimal biological dose. The study will start at Cohort A (NKTR-255 starting on day 14 at 1.5 µg/kg), then escalate to Cohort B (NKTR-255 starting on day 10 at 1.5 µg/kg) if not toxicities are observed. In the event of DLTs, the dose of NKTR-255 may be de-escalated to Cohort C (0.75 µg/kg starting on day 14). After NKTR-255 administration is deemed safe in Cohorts A and B, the expansion cohort may commence where up to 24 subjects may be enrolled in total. The proposed recommended dose was based on the starting dose of the first-in-human phase 1 study of single agent NKTR-255 in patients with R/R hematologic malignancies (NCT04136756). The dose limiting toxicity (DLT) period is 21 days after the first NKTR-255 dose. The primary objectives include safety and tolerability of NKTR-255 in combination with liso-cel, optimal biological dose (OBD) determination, and complete response (CR) rate at 3 months. Secondary endpoints include CR rate and objective response rate (ORR) at 6 months, duration of response (DOR), progression free survival (PFS), and overall survival (OS). Up to 24 patients will be enrolled during cohort allocation to determine the OBD. Clinical trial information is available at CT.gov: NCT05359211.
Conclusions: This clinical trial will evaluate the safety and efficacy of NKTR-255 in combination with liso-cel aiming to increase the response rates and durability of response compared with CD19 CAR-T cells alone. Based on the biological rationale of IL-15 supplementation, correlative data from CD19 CAR-T cell studies, and results from preclinical studies with xenogeneic mouse models, NKTR-255 has the potential to augment currently approved cellular therapies.
Disclosures
Vinaud Hirayama:Juno Therapeutics, a BMS Company: Research Funding; Novartis: Honoraria; Nektar Therapeutics: Research Funding; Bristol Myers Squibb: Honoraria. Chou:Genentech: Current Employment. Maloney:Celgene: Consultancy, Honoraria, Other: Data Safety Monitory Board , Research Funding; Bristol Myers Squibb: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Legand Biotech: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Mustang Bio: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; A2 Biotherapeutics: Current holder of stock options in a privately-held company; Fred Hutch: Patents & Royalties: For Patients licensed to Juno ; Bioline Rx: Membership on an entity's Board of Directors or advisory committees, Other: Data Safety Monitory Board ; Umoja: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Navan Technologies: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Incyte: Consultancy, Honoraria; A2 Biotherapeutics: Consultancy, Honoraria. Marcondes:Nektar Therapeutics: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Turtle:Juno Therapeutics, a BMS Company: Patents & Royalties, Research Funding; Expert Connect: Consultancy; Allogene: Membership on an entity's Board of Directors or advisory committees; Kite Pharma, a Gilead Company: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Decheng Capital: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eureka Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Precision Bioscience: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; T-CURX: Membership on an entity's Board of Directors or advisory committees; Caribou Bioscience: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Century Therapeutics: Membership on an entity's Board of Directors or advisory committees; Arsenal Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Prescient Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
*Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal